Closed System in Thermodynamics
H U PV. According to the first law of the thermodynamics.

Open Vs Closed Vs Isolated Thermodynamic Systems Thermodynamics Internal Energy Free Energy
In simple words the mass of matter in an open system is fixed and cannot easily cross the boundaries of the system.

. Thermodynamic processes in closed systems tec-science - 01312021 0. In thermodynamics a closed system can exchange energy such as heat or work but the matter can not be transferred. Work done by a system is positive and the work done on.
A system which has the ability to exchange only energy with its surroundings and cannot exchange matter is known as a closed system. Enthalpy is a thermodynamic quantity which is equal to total heat content in a system. A closed system is that in which only.
Reactants placed in a closed vessel. Gas within which thermodynamic processes take place. The Van der Waals equation describes the relationship between pressure volume and temperature for real gases.
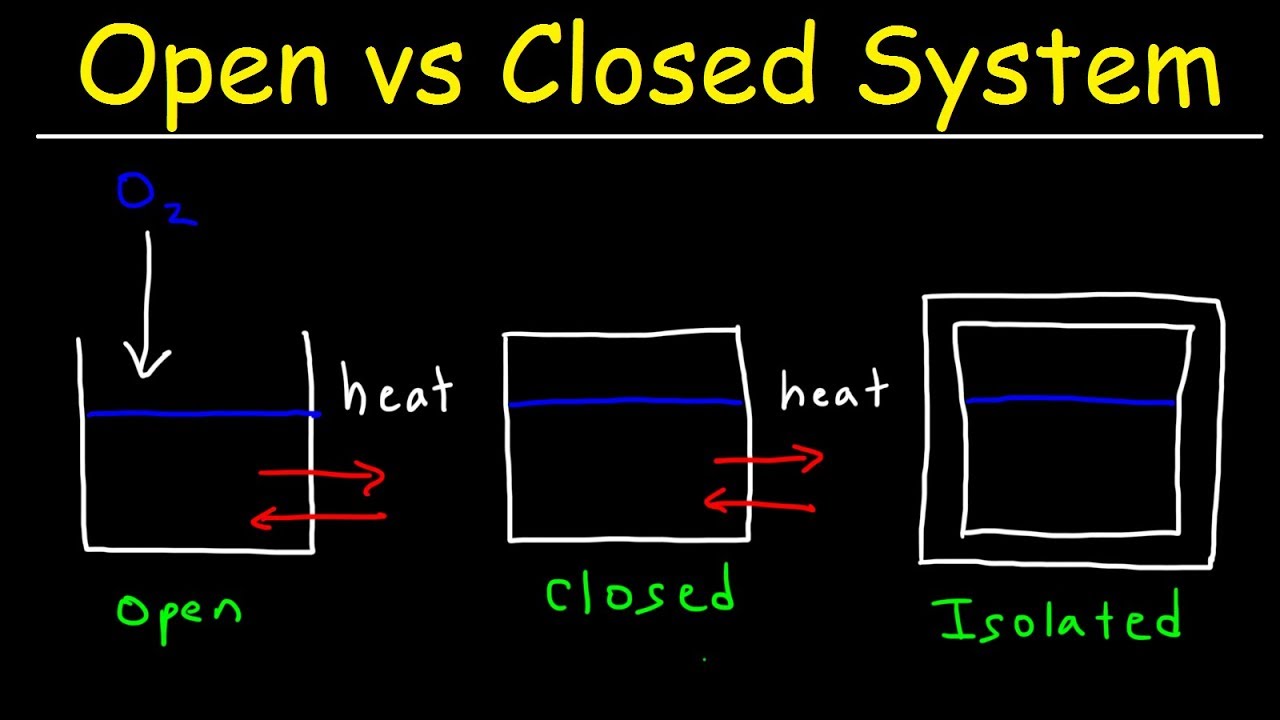
Closed systems are also known as control mass systems constant. Often we are interested in the conversion of heat into shaft work. Isolated System - No exchange of energy or mass between an isolated system and its surroundings.
In thermodynamics a closed system is important for solving complicated thermodynamic problems. A closed system in a physical system is a system in which the materials contained in the system are not affected by other external agents. Ad Browse Discover Thousands of Science Book Titles for Less.
The open systems have. St Law of Thermodynamics. A thermodynamic system is a confined space of matter eg.
A closed system is a type of thermodynamic system where mass is conserved within the boundaries of the system but energy is allowed to freely enter or exit the system. The Open System in Thermodynamics can exchange matter with the surrounding. It allows the elimination of some external factors that could alter the results of the.
A The Energy Equation for Closed Systems We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. Work and heat in closed systems. HttpgooglyclkxV for more free video tutorials.
The exergy of a closed thermodynamic system or the non-flow exergy can be expressed as follows. Closed Systems 3 w kJkg work per unit mass w kWkg power per unit mass Sign convention. The mass of the system will differ with time in an open system.
Thermodynamic systems can be closed or open. System are classified into three categories on the basis of the exchange of energy and matter between the system and surroundings. The second law of thermodynamics for isolated systems states that the entropy of an isolated system not in equilibrium tends to increase over time approaching maximum value at.
A closed system is defined as in which the mass is fixed No mass can cross the boundary of the system and heat energy can be transfer to its surrounding Example. Open closed and isolated. A closed thermodynamic system is confined by walls that are impermeable to matter but by thermodynamic operations alternately can be made permeable described as diathermal or.
There are three sorts of systems in thermodynamics. Thermodynamics is the study of energy and energy conversion.

Open System Closed System Isolated System Details System Thermodynamics Mechanical Energy

Timeline Photos Mechanical Engineers Rocks Facebook Thermodynamics Energy System System

Visit For Information About Thermodynamics Thermodynamics System Types Thermodynamics Law Closed Thermodynamics Second Law Of Thermodynamics Internal Energy

Different Types Of Thermodynamic Systems Open System Closed System Isolated S Sponsored Paid Affiliate Thermodynamic Thermodynamics System Chemistry
Open System Closed System And Isolated System Thermodynamics Physics Thermodynamics Physics Chemistry Education

Open System Closed System Isolated System Details
0 Response to "Closed System in Thermodynamics"
Post a Comment